Protons, neutrons, electrons, atoms…ahhhh! They are the foundation of understanding chemistry, but oh so easily confused. So, in an effort to make at least one foundation clear, let’s talk about electrons and their charges. You probably have some basic questions about electrons that we’re going to answer for you.
Are electrons positive or negative?
How do we find the number of electrons in an element?
More importantly… why does any of this even matter?
Before we answer these questions, let’s briefly learn about atoms and how they’re related to electrons.

What is an atom?
In basic terms, atoms are the basic building blocks of matter. Matter is anything that we can physically touch. While cells are the foundation of all living things (link to post about plant cells!), atoms go beyond life and make up the entire world around us, both living and non-living.
This means atoms are unable to reproduce themselves. Rather than thinking in biological terms (like cells), think of atoms as a chemical composition. Within that composition are three main subatomic particles:
- protons
- neutrons
- electrons
The protons and neutrons hold their spot in the center of the atom, also known as the nucleus. Meanwhile, electrons orbit this nucleus within an electron shell — sometimes likened to as an orbital or cloud. Think of it as the planets orbiting the sun; protons and neutrons are the sun, and electrons are the planets!
The mass of an atom is almost solely derived from the nucleus. However, the nucleus only takes up approximately 1/10,000 of the available space within an atom. This leaves a lot of empty space for the electrons to orbit. Speaking of mass, electrons are around 1/2000 of the mass of a proton or neutron.
You’re probably wondering if we can view atoms with the naked eye. Nope! It takes a very special microscope to study atoms and their inner workings. That being said, there is still a lot that has been learned about atoms, with new information being discovered every day!
So, there’s a very brief overview of atoms. For visual learners, check out this fun video about the basics of atoms and what they’re made up of. Now let’s dive into the world of electrons and their charges!
Are electrons positive or negative?
Electrons are negatively charged particles. Whereas, protons are positively charged. Cool info, but what does this actually mean? Well, in the same way that atoms are the basic building blocks of matter, electric charge is a fundamental property of matter. This electric charge is the result of either a surplus or scarcity of electrons. So, because electrons have a negative charge (-1), atoms with more electrons than protons have an overall negative charge and are called anions. Atoms with a positive charge (more protons than electrons) are called cations.
Negative and positive charges are attracted to each other. Turns out that opposites really do attract! So, electrons are attracted to protons and vice versa. Like charges repel each other.
How to Find the Number of Electrons in an Element
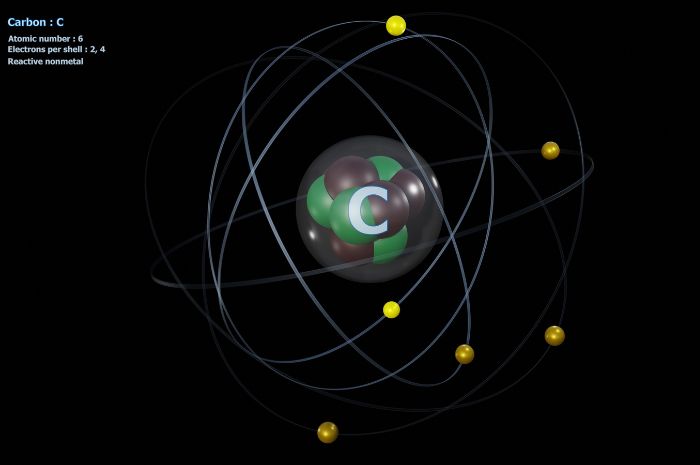
Finding the number of protons in an element is easy; just look at the atomic number on the periodic table of elements! Finding the number of electrons in an element is just a bit more complicated. If the atom is neutral, the number of electrons is the same as the number of protons. Easy peasy.
If the atom is positively charged, however, we subtract the charge number from the number of protons to get the electron count. Vice versa, if the atom is negatively charged, we add the charge number to the atomic number to get the electron count.
For example, how many electrons does carbon have? Well, if we look at the periodic table, the atomic number is six. This means that there are six protons in the carbon atom. In its neutral state, carbon will have six electrons as well. But let’s say carbon has a +2 positive charge. This means there will be four electrons in that specific carbon element. If the carbon had a -2 charge, there would be eight electrons.
If this doesn’t make sense just yet, check out this super helpful article providing more examples of elemental and electron charges.
Electrons in Our Daily Lives
Even though we can’t actually see the movement of electrons within an atom, we benefit greatly from this movement! Electricity – what powers our lights, our phone chargers, and SO many other aspects of our daily lives – is a result of the movement of electrons between atoms! Sometimes, electrons are pushed out of their electron shell and shifted to another atom. This seemingly simple process is what creates electricity.
Have you ever watched a big bolt of lightning flash in the sky? Lightning is electricity! The electrons in one cloud are moving to another cloud. When we see lightning strike an object (oftentimes a tree), that is a result of electrons jumping from the cloud to the ground. How incredible is it that such tiny electrons can create such a grand gesture of force on Earth?
If you happen to have a balloon, blow it up and then rub the balloon on the top of your head. Did your hair go crazy and stick straight up? That is static electricity! Electrons transferred from the balloon to your hair. As the electrons move, they repel each other. This creates the movement of your hair from flat to sticking up!
Want to learn more about electrons and their charges?
Chemistry often gets a bad rap for being complicated and boring. At Journey Homeschool Academy, we’re here to tell you that it doesn’t have to be this way! With Experience Chemistry, we bring the fun back into high school chemistry! Through engaging video instructions, extra cool labs, and helpful study guides, your high schooler will not only learn but learn to love even the complicated parts of chemistry!
Electrons and their charges are only scratching the surface of the wonders of chemistry. Dive deeper and watch your student marvel at electrons, neutrons, and so much more!
