Experience Chemistry (Level C)
Full-year course: $279
Give your high school student a complete, hands-on chemistry education without the overwhelm.
Experience Chemistry (Level C) combines engaging video lessons, guided labs, and structured assignments to help students explore the foundational principles of chemistry while strengthening problem-solving and analytical thinking skills.
Every concept is presented from a Christian worldview, so your teen learns to see God’s order and design in every atom and reaction—no parental science expertise required.
✔️ Video Lessons
✔️ Lab Assignments
✔️ Quizzes & Exams
✔️ Reading Assignments
Everything you need for a complete science experience:
- 35 weeks of engaging video lessons — 2 videos with every weekly lesson
- Lab Manual with weekly laboratory experiments and activities*
- Student Guidebook with fill-in-the-blank outlines and study guides*
- Weekly homework questions or practice equations to deepen understanding of scientific concepts
- Weekly comprehension quizzes
- Exams to gauge your student’s understanding
- Lab videos to walk your student through laboratory exercises
- Optional reading assignments from our recommended textbook Modern Chemistry PLUS alternative reading lists for several other textbooks
- Course guide
- Certificate of completion
- Start anytime and get 12 months of access
*Manuals and guidebooks are included as a PDF download with the purchase of your course. Physical copies available to purchase separately.
Scope & Sequence
This honors-level high school chemistry course provides an in-depth exploration of fundamental chemical principles and their applications. Through a structured series of lessons, students will engage in hands-on activities, laboratory experiments, and problem-solving activities to develop a robust understanding of matter, measurements, atomic structure, the periodic table, chemical bonding, reactions, stoichiometry, gases, and thermodynamics. This curriculum prepares students for continued study in chemistry and related fields at the college level.
Lesson 1: Introduction to Chemistry
-
What is Chemistry?
-
The Five Branches of Chemistry
-
The Scientific Method
Lesson 2: Matter, Measurements, and Unit Conversions
-
Matter and mass
-
SI Unit System: base units and prefixes
-
Unit Conversions between metric units using the factor label method
-
Units of temperature and conversion
Lesson 3: Accurate and Precise Measurements in Science
-
Accuracy and precision
-
Understanding and using significant digits
-
Scientific Notation
-
Density Problems
Lesson 4: The Atom
-
Scientific models
-
The history of atomic models and influential scientists
-
Physical and chemical properties
-
Physical and chemical changes
Lesson 5: Energy!
-
Energy and chemistry
-
Exothermic and endothermic reactions
-
States of matter and molecular energy
-
Compare and contrast pure substances and mixtures
-
Pure substances: elements and compounds
-
Mixtures: homogeneous and heterogeneous
-
Suspensions and colloidal dispersions
Lesson 6: Introduction to the Periodic Table
-
History of the Periodic Table
-
Mendeleev
-
Elements and their symbols
-
How we use the periodic table: chemical symbols, mass numbers, atomic numbers, and element name
-
Atomic Mass Units
-
Defining Periods and Groups
-
Valence electrons
-
Finding the number of protons, neutrons, and electrons for elements
-
Isotopes
Lesson 7: Periodic Chemical Properties
-
Identifying periods, families, and series on the Periodic Table
-
In-depth Look at the Periodic Table
-
Unique Family Trends on the Periodic Table
-
Unique Period Trends on the Periodic Table
-
How periodic trends help predict reactivity
Lesson 8: Electrons (Part 1)
-
Relating electrons to sublevels and energy levels
-
Electron orbital notation and configurations
-
Valence Electrons and why they are important
-
Writing Electron Dot Structures
Lesson 9: Electrons (Part 2)
-
Valence Electrons and why they are important
-
Writing Electron Dot Structures
-
The Octet Rule
-
The 3 Types of Chemical Bonds: Ionic, Covalent, and Metallic
-
Electronegativity and Bonding
-
Polarity
Lesson 10: Introduction to Chemical Formulas
-
Molecular chemical formulas
-
Subscripts and coefficients in formulas
-
Counting atoms
-
Hydrogen: a unique element
-
Water and its unique properties
Lesson 11: Exam 1
Lesson 12:
-
Defining oxidation numbers
-
Assigning oxidation numbers using the 6 Rules
-
Polyatomic Ions
-
Multiple oxidation numbers
-
Comparing structural, molecular, and empirical formulas
Lesson 13:
-
Naming compounds from their formulas
-
Writing formulas of compounds from their names
-
Using Greek prefixes in chemical names
-
Multiple oxidation states when naming
-
Identifying and naming acids
Lesson 14: Balancing Chemical Equations
-
Examining chemical equations
-
What is a balanced equation?
-
How to balance chemical equations
Lesson 15: Chemical Reactions
-
Identifying reactions: combination, decomposition, single replacement, and double replacement
-
Combustion reactions
-
Acid-base neutralization reactions
-
Oxidation-reduction reactions
Lesson 16:
-
Moles in chemistry
-
Avogadro’s number
-
Converting units with moles and stoichiometry
Lesson 17:
-
Empirical formulas
-
Percent composition
-
Gram atomic mass and gram molecular mass
Lesson 18:
-
More stoichiometry: finding more values with the mole
-
Convert from moles of one substance to moles of another
-
Convert mass to moles for given substances
-
Convert from mass of one substance to mass of another substance
-
Limiting reactant
-
Theoretical and percent yield
Lesson 19: Exam #2
Lesson 20: Kinetic-Molecular Theory
-
Kinetic-molecular theory and properties of gases
-
Physical properties of gases
-
How temperature and pressure affect the volume of a gas
-
Standard temperature and pressure
Lesson 21: States of Matter
-
Characteristics of solids at the atomic level
-
Characteristics of liquids at the atomic level
-
Characteristics of gases at the atomic level
-
Plasma
-
Phase changes and reading phase change diagrams
Lesson 22: Gas Laws (Part 1)
-
Boyle’s Law to find pressure or volume of a gas
-
Charles’ Law to find volume or temperature
-
Avogadro’s Law and kinetic-molecular theory
Lesson 23: Gas Laws (Part 2)
-
Dalton’s Law of Partial Pressure and related equations
-
Molar volume relationships and gas volume at STP
-
Finding the gram-molecular mass of gas using density
-
Ideal gas law to find pressure, volume, moles, and temperature
Lesson 24: Phase Transitions
-
Factors affecting the rate of evaporation
-
Diffusion
-
Vapor pressure
-
Plasmas
-
Using a phase diagram to make predictions about the states of matter
-
Concepts of heat capacity, Heat of Fusion, and Heat of Vaporization
Lesson 25:
-
Dipole-dipole forces, hydrogen bonds, and dispersion forces
-
Predicting types of forces between molecules
-
Differences in physical properties in types of bonding and forces present
-
Using kinetic theory to explain properties of solids
-
Crystalline and amorphous solids
-
Kinetic Theory and state changes: melting, freezing, boiling, evaporation, sublimation, condensation
Lesson 26: Exam #3
Lesson 27:
-
Kinetic theory and properties of liquids
-
Solutes and solvents
-
Surface tension, viscosity, capillary action, diffusibility, and permeability
-
Process of dissolving
-
Why certain solutes do not dissolve in certain solvents
-
Electrolytes
-
How pressure affects boiling
Lesson 28:
-
Identifying types of solutions by their descriptions
-
Techniques that increase solubility
-
Defining concentration
-
Concentration calculations
Lesson 29:
-
Explain how solutes affect the colligative properties of a solution
-
Concentration increases and vapor pressure, freezing point, and boiling point of a solution
-
Calculating boiling point elevation and freezing point depression problems
Lesson 30:
-
Process of osmosis
-
Colloids
-
Differences between suspensions, solutions, and colloids
-
The Tyndall effect
Lesson 31:
-
How thermodynamics impacts chemistry
-
Understanding calorimeters
-
Distinguishing between temperature and heat
-
How states of matter relate to sensible heat and latent heat
-
Exothermic and endothermic reactions
-
Defining specific heat
-
Enthalpy and Entropy
Lesson 32:
-
Using energy diagrams
-
Understanding the kinetics of reactions
-
How activation energy affects reactions
-
Applying the collision theory to reaction rates
-
Different factors that influence reaction rates
-
How enzymes work
Lesson 33: Acids & Bases
-
Classifying acids and bases using Arrhenius, Bronsted-Lowry, or Lewis definitions
-
Properties of acids, bases, and salts
-
Classifying solutions as acidic, basic, or neutral
-
Convert between pH, pOH, (H₃O⁺), and (OH⁻)
-
Acid-base indicators
Lesson 34:
-
Equations for neutralization reactions between acids and bases
-
Completing equations for neutralization reactions between acids and bases
-
Performing titrations and the information they provide
-
Calculating concentration of a solution from given titration data
-
Buffer systems
Lesson 35: Exam #4
Full-year course: $279
- Pay in full or in 3 monthly installments
- Generous multi-student discounts available for families with Level B & C students taking the same course in the same year
- Educational Savings Accounts (ESA) available in many states
Trusted by more than 18,000 homeschool families worldwide
See why so many parents choose Journey Homeschool Academy for hands-on, faith-based science.
My son had the choice of taking a co-op class or another JHA class to finish out his senior year, and he said hands down, Experience Chemistry was his first choice!
I have taken many college level science classes that never explained the same material in such a straight forward, easy to understand way. The quality of the instruction is thorough and tackles even the most challenging ideas with wit and humor. It was never work to get my son to listen to your lectures, he enjoyed them so much. When we look back on our last years of homeschooling, your classes will be one of the delightful highlights we’ll remember.
~Wendy
I have an engineering degree, but my kids don’t want to hear me drone on about science all day…
Experience Chemistry frees parents up and gives students the confidence to perform labs all by themselves. The labs have an intro video explaining how the lab works before the student has to attempt it on their own. This takes a lot of pressure off the student and parent.
~Patricia
I love that I don’t have to remember anything from my high school chemistry class 30 years ago! My daughters are learning far more than I could have ever taught them if I’d tried to teach it on my own. I was fine for teaching my daughters elementary and middle school science, but I can’t even imagine trying to teach Chemistry on this level.
~Bev
After completing Experience Biology, I was afraid that Experience Chemistry would be boring and the concepts difficult to understand. But Experience Chemistry had all the tools I needed to be successful. It has videos that helped me feel connected to the teacher, reading that reinforced what I was learning, and homework questions that challenge me to apply what I’d learned. I also loved how the lessons applied science to real world examples!
~Alina O.
I see so many people put their kids back in school when they get to high school, because they lack the confidence to teach courses like chemistry. Thanks to you guys, that is no longer an excuse. The videos in this course are top notch and I like the layout of the workbook with the opportunity it provides to learn notetaking skills. This is a challenging course, designed well, and taught from a biblical worldview. Thank you for working so hard to put these amazing courses together!
~Jennifer G.
Having kids in high school now, we were looking for a faith-based class that would count for credit with our school district’s homeschool program. We like the hands-on labs in this program and appreciate the history and Bible references throughout. While Experience Chemistry has been challenging, it’s been a good experience for our family!!
~Beth
You’ve got questions… we’ve got answers
What’s included in my purchase?
You’ll have access to all lesson and lab videos along with PDF downloads of the student guidebook, lab guide, curriculum guide, answer keys, homework help videos, and sample lab reports.
Should you want a physical copy of the guidebook or lab guide, those are available for purchase in our store. It is highly recommended that you purchase the textbook Modern Chemistry.
You will also be responsible for purchasing lab supplies for any labs you’d like your student to complete.
Are there any math prerequisites for this course?
Your student should have completed Algebra 1 prior to taking this chemistry course. This is a rigorous math-based chemistry course.
When should my student take this course? What grade?
Students in the 11th and 12th grade have had the most success when taking this course. There is a lot of math and the course moves at a fast pace to cover all of the material. Many parents choose to list this as an honors course on their student’s high school transcript.
If you are looking for a class for younger students or students you’re not sure would be able to keep up with the pace of this course, we recommend checking out our Astronomy, Biology, or Earth Science courses.
When does the course begin?
You’ll have immediate access to the course as soon as you register!
Since this course is self-paced, you, the parent, have control over when your student begins the course. You can start as soon as you register, or wait a few weeks or months. Beginning the course is as easy as pressing a button. Once you begin the course for your student, they will have one year to access the course.
How long will I have access to this course?
You’ll have access to the course for one year of access — you decide when your student begins the course. There are 35 weekly lessons, so that should provide ample time for your student to complete the course. Once each video is live, your student can watch and re-watch it anytime they would like to throughout the duration of the course.
Does my child have to view the course videos on specific days or times?
No, students can watch the lessons any time throughout the week. Once released, each video can be reviewed as many times as the student wants through the end of the course.
Can my student earn high school credit for this course?
In most states, students need to accumulate at least 120 hours of instruction and course work to receive a high school credit.
Many parents choose to record Experience Biology as an honors level lab-science on their student’s high school transcript. We recommend your student complete all of the following to accumulate the necessary course hours for high school credit:
- complete all the course lectures
- take all the quizzes and exams
- complete recommended readings
- watch laboratory videos and complete a minimum of 15 lab assignments
How you use this course and the resources available is ultimately up to you, the parent, as this is a homeschool course.
Can I enroll more than one student in this course at the same time?
In the upper level course, each student receives their own grade, online portal, and auto-graded quiz so each student should enroll separately. We do offer generous multi-student discounts for families enrolling more than one student in the class at the same time.
Can we use a different textbook for this course?
We believe parents are best equipped to determine their students’ educational needs. As a homeschool course, you’re welcome to choose whatever textbook you’d prefer for your student to use or omit textbook reading altogether if you’d prefer. For most students, anytime they can use multiple modes of learning it helps them retain information better.
After reviewing many, many textbooks we chose Modern Chemistry because we believe this textbook does an excellent job of reinforcing the concepts students are learning in the lectures. We provide recommended reading for your student from this book. If you choose to use another textbook, you’ll need to be sure to assign students what you’d like them to read each week.
We have also created alternative reading schedules to accompany several other commonly used textbooks by homeschoolers. While we cannot vouch for every detail in these textbooks, the reading lists provided here generally overlap with what is covered in each of the lessons in the course.
In addition to our recommended textbook Modern Chemistry, we currently have a reading list available for the following textbooks:
- Discovering Design With Chemistry, Berean Builders (1st edition)
- Exploring Creation With Chemistry, Apologia (3rd edition)
- Chemistry, BJU Press (5th edition)
- Chemistry: Precision and Design, Abeka (3rd edition)
- General Chemistry, Novare (2nd edition)
- Conceptual Chemistry, Pearson (5th edition)
How long do students have to complete individual lessons?
We recommend students plan to complete one lesson per week to finish the course during a typical school year. Once your student begins the course they will receive access to the next lesson within 1-2 hours of completing the current lesson. This allows you the flexibility to work ahead if needed to account for vacations or time off while still finishing the course in the typical timeframe. Students will have a full calendar year to complete the course, so if they fall a week or two behind, they should still have plenty of time to complete the course.
Can I take this course if I live outside the U.S.?
You sure can! We’ve made sure to make the lessons applicable to those worldwide.
What additional books or supplies will I need for this course?
Students will need a number of different supplies to complete the labs and activities work. See the complete Experience Chemistry Lab Supply List.
It is highly recommended, though not required, that you purchase the Modern Chemistry textbook. While this is our preferred textbook for the class, we’ve also included reading lists for several other textbooks, or you may omit textbook reading.
A PDF download of the Student Guidebook and Lab Guide are available as part of the course purchase.
Can I pay for this course with my Educational Savings Account (ESA)?
We are an approved vendor with several ESAs and are continuing to add to the list all the time! We’re happy to provide you with invoices or other information you need to submit to your ESA. You can see all the ESAs we’re currently approved vendors with and/or submit a request for us to apply to the ESA in your state.
Do you offer options for using this course with a co-op?
We offer co-op pricing for groups of 4 or more who have designated a single instructor/ facilitator. Co-op courses are designed for groups that are meeting at least weekly.
Our co-op courses are set up differently than our family programs to accommodate groups. You can learn more about our co-op courses here.
Please note: because of the way our group programs are set up, we are not able to communicate with individual parents of students within co-ops.
What is your refund policy?
We offer a 100% money-back, happiness guarantee within 30 days of the course start date OR 1 year from purchase date (whichever isless). Simply email us and we’ll give you a full refund.
Understanding the Fabulous Fit Promise
We want this course to be a fabulous fit for your family. Based on our parent and student feedback, we think you will be.
However, we offer a 100% money-back, happiness guarantee within 30 days of the course start date OR 1 year from purchase date (whichever is less).

Two engaging video lessons for every weekly lesson to help your child understand new and exciting concepts without feeling overwhelmed.

Carefully planned lab work with corresponding lab video lessons throughout the year so your child can experience chemistry — hands on! You can see the lab supply list here.

Online, graded quizzes and exams, so you can take a break from correcting your child’s work.
Homework questions & practice equations to help your student apply the rich concepts covered in this course.
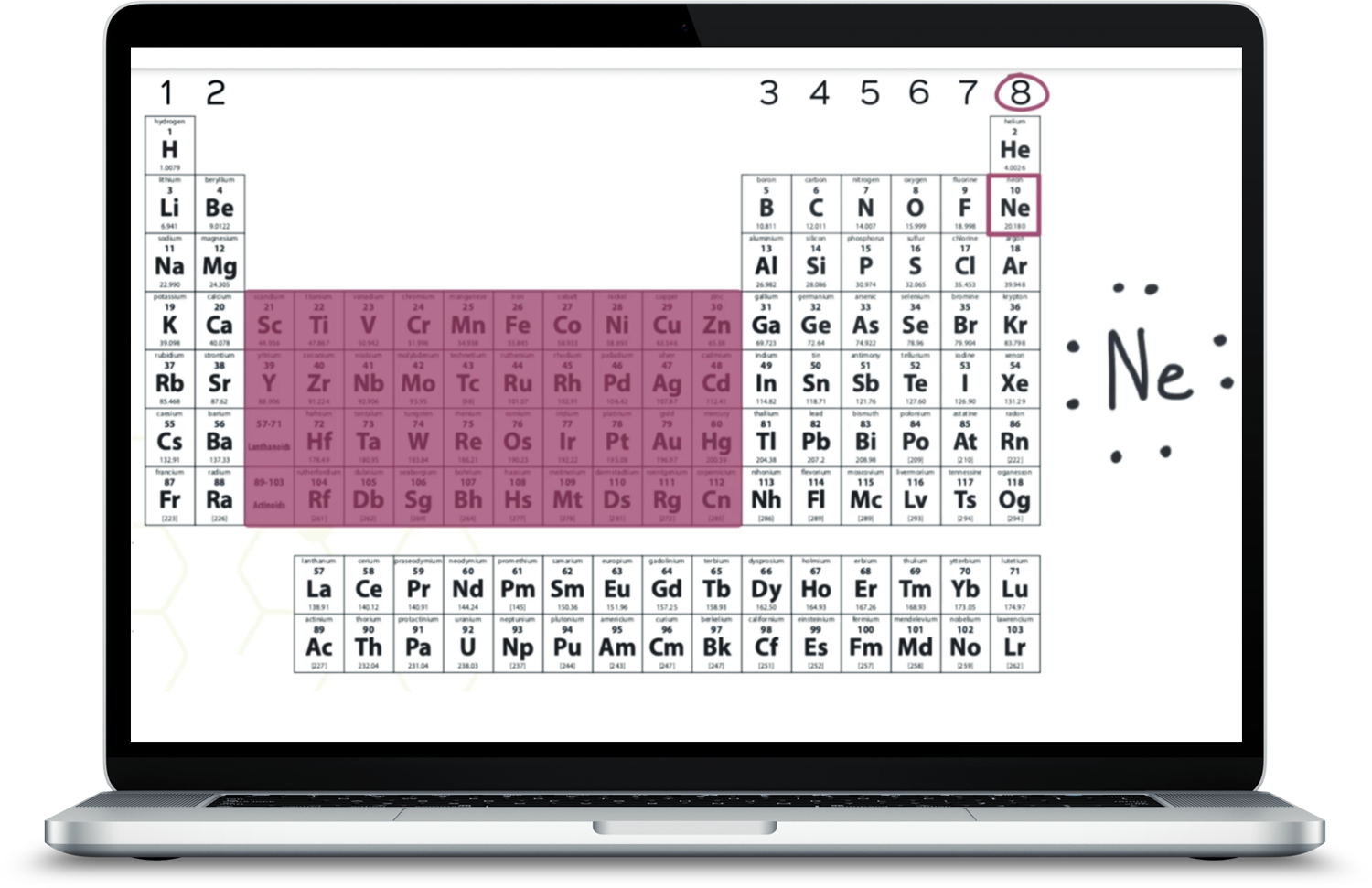
Homework help videos to assist your student when they get stuck! We walk them through the tricky math and chemical equations step-by-step.
Lesson outlines to help your student grasp important concepts, learn new terms, and help with note taking.

Access your lessons on any device.

Reading assignments that will help your student further their understanding of the course material in a new way.

Certificate of completion for a job well done when the course is completed.

